AMAZON multi-meters discounts AMAZON oscilloscope discounts
No guide on power supplies would be complete without a discussion of batteries as a source of power for electronic circuits. In this chapter we will take a look at the various types of batteries, their application to electronic circuits, their advantages, and their limitations.
Before launching into our discussion of batteries, there are several basic points to keep in mind. First, the term "battery" is frequently used incorrectly. By definition a battery is a source of d-c power consisting of two or more chemical, nuclear, solar, or thermal cells. A single unit such as is common referred to as a flashlight "battery" is actually a "cell"; if two or more cells are connected together, they form a battery of cells, or "battery." A 12-volt auto storage battery, for example, consists of six 2-volt cells connected in series to provide a total of 12 volts.
There are two basic types of cells: primary and secondary. The primary cell is exhausted after its internal chemicals have been consumed in their electricity-producing chemical reaction. Normally one or more of the products of the reaction are lost. On the other hand the secondary cell may be reactivated, when it is exhausted, by passing direct current through it. This current, in the opposite direction from that originally developed by the cell, reverses the electrochemical action and restores the chemicals to their original state.
PRIMARY-CELL OPERATION
The first practical primary cell was produced in the 1800's by Volta, an Italian scientist. He discovered that two dissimilar metals immersed in an electrolyte start a chemical reaction which produces a potential difference between the two metal electrodes. A typical voltaic cell, as shown in FIG. 1, consists of a strip of copper and one of zinc, placed in an electrolyte made up of a weak sulfuric-acid solution.
If current is to be produced, there are certain requirements which must be met in the primary cell. These include:
1. The two electrodes must be made of dissimilar materials.
2. The electrolyte must be an acid, a salt, or an alkali that will react with the electrodes.
3. The electrolyte must be a good conductor of electricity.
There are certain definite characteristics that are common to all types of primary cells. The current that can be delivered by a cell is primarily determined by the active area of its electrodes--where the electrode and the electrolyte can react. The terminal voltage developed by the cell is not determined by the size or spacing of its electrodes, but rather by the materials used in its construction.
To understand the basic electrochemical action of a simple primary cell, refer again to FIG. 1. In a weak solution the sulfuric acid (H2S04) breaks down into three charged particles. The sulfate portion takes on two additional electrons to become a negatively charged sulfate ion (S04=). The two hydrogen atoms lose electrons to become two positive ions ( H +). As the zinc strip dissolves in the sulfuric-acid electrolyte, zinc ions (Zn+) enter the solution, leaving free electrons from their valence rings on the zinc electrodes. The zinc electrode now has a net negative charge due to its surplus of free electrons.
The chemical reaction between the electrolyte and copper electrode is basically the same, although not as intense. Thus, while the copper electrode also develops a negative charge, it is not so large as that produced on the zinc electrode. Since the latter is more negative than the former, a voltage difference exists between the zinc and copper electrodes.
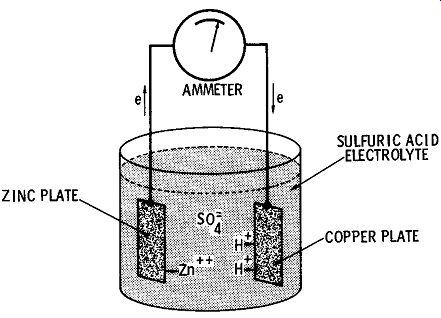
Fig. 1. Simple primary cell.
When a conductor is provided between the copper and zinc electrodes, the surplus of free electrons on the zinc electrode flow to the less negative copper electrode. Here hydrogen ions combine with electrons to form hydrogen gas on the surface of the copper electrode.
Thus there is a complete electrical circuit; positive zinc ions are formed at the zinc plate at the same time positive hydrogen ions leave the solution, and electrons move through the conductor from the zinc plate to the copper plate. The action continues until the surface of the copper electrode is completely covered with hydrogen gas. When this point is reached, hydrogen ions cannot approach the copper plate, so electrons no longer flow. The cell is said to be "polarized." While the simple zinc-copper-sulfuric-acid cell serves to illustrate the basic electrochemical action of a primary cell, it leaves much to be desired from a practical standpoint. Back in 1880, the first practical primary cell was developed by a Dr. Gassner. Because its electrolyte is in the form of a paste rather than being a liquid, it is termed a "dry cell." FIG. 2 shows the internal construction of a typical dry cell. The outer zinc can serves as both the negative electrode and the container for the mix core ( paste electrolyte). The can is produced from special zinc that is 99.9 percent pure. The positive electrode consists of a carbon rod placed in the center of the zinc can. The area between the carbon rod and the zinc shell is filled with the paste electrolyte consisting of manganese dioxide, acetylene black, ammonium chloride, zinc chloride, chrome inhibitor, and water. The manganese di oxide serves as a depolarizer, absorbing the hydrogen gas as it ac cumulates around the carbon rod ( positive electrode) during periods of discharge. The open-circuit voltage developed by a carbon-zinc dry cell is approximately 1.5 volts.
The exact details of the internal construction of the zinc-carbon cell can be varied depending upon its intended use. For example, for flashlight service a cell must be capable of supplying a current of approximately 0.25 to 0.5 ampere, depending upon the type of bulb employed. Periods of usage may range from several seconds to several minutes at a time. Cells designed for photoflash service must de liver a high surge of current ( in the range from 2 to 10 amperes) lasting less than one second. On the other hand, cells designed for transistor-radio service must furnish a relatively steady current of 10 to 100 ma for several hours. Proper design of the dry cell can provide optimum service for each application.
Environmental temperature plays an important role in the performance of a zinc-carbon cell. Optimum operating temperature for the zinc-carbon cell is approximately 70°F. Prolonged exposure to temperatures above 130°F may cause premature failure of the cell. Cells operating at temperatures of 0°F or lower will have their current capability greatly decreased. This is due to the fact that the chemical action within the cell is greatly retarded at low temperatures. On the other hand, storage of zinc-carbon cells at low temperatures will ex tend their shelf life because the chemical reaction that takes place even when the cell is not connected is slowed. Zinc-carbon cells can be stored for a number of years with little sign of deterioration at temperatures near 0°F. (They must not be allowed to freeze, how ever.) High-temperature storage greatly reduces their shelf life since the chemical action is accelerated and moisture is lost from within the cell.
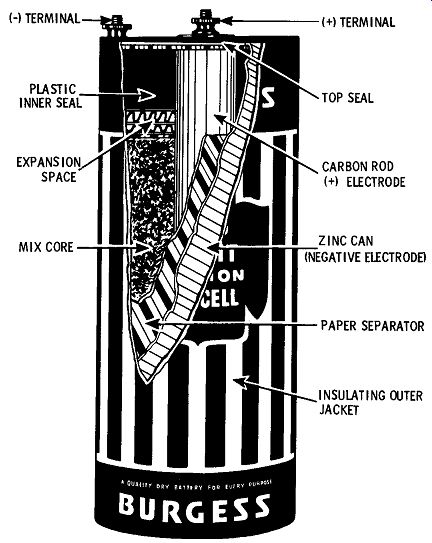
FIG. 2. Construction of a carbon-zinc dry cell.
INTERNAL IMPEDANCE
All cells have a certain internal impedance which may be considered as a resistance placed in series with the cell when it is in a circuit ( FIG. 3). The effect of this resistance is to limit the amount of cur rent which a cell can deliver to an external load. As the current in creases, the voltage drop across the internal impedance also increases, reducing the voltage available at the terminals.
A fresh carbon-zinc cell will exhibit a low internal resistance ( a fraction of an ohm). During its period of service, the internal impedance gradually increases, causing the voltage across its terminals to drop with the application of the load. As the cell nears the end of its useful life, its internal impedance will be so great that its output voltage will drop to an unacceptable value when a load is applied. Be cause of this internal impedance, the terminal voltage of a cell should always be checked with the normal load connected. Without a load the terminal voltage will be at its maximum, giving an erroneous indication that the cell is still in satisfactory condition.
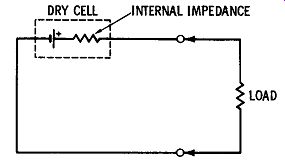
FIG. 3. Internal impedance of a cell.
MERCURY CELLS
The mercury cell is capable of providing the most stable output voltage over a given period of time of any conventional primary cell.
The terminal voltage of the mercury cell is 1.350 volts plus or minus one-half of one percent. As indicated by FIG. 4, this voltage drops less than one percent of its initial value after the cell has been stored for several years. Mercury cells exhibit excellent shelf-life characteristics; cells over 14 years old are practically as good as new. Because of this, the mercury cell is often employed as an inexpensive secondary-voltage standard.
FIG. 5 illustrates the relatively flat voltage-decay characteristic of a typical mercury cell. Notice that its voltage remains nearly constant over most of its operating life when used with relatively light loads.
The basic mercury cell ( FIG. 6) consists of an amalgamated zinc anode formed from highly purified compressed zinc powder, a cathode of mercuric oxide, and an electrolyte consisting of potassium hydroxide. The cathode and anode are separated by a porous material which has absorbed the electrolyte. It is possible for ions to flow between these two electrodes.
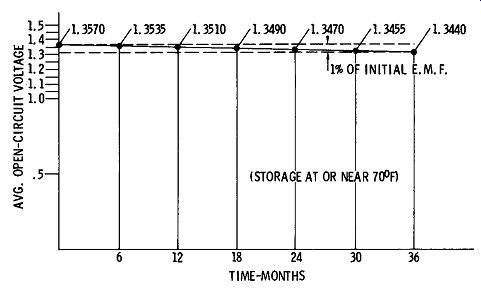
FIG. 4. Voltage of mercury cells after storage .
Some mercury cells employ a depolarizer consisting of a small amount of manganese dioxide; the initial no-load voltage of these cells is 1 .45 volts. This higher value of voltage drops shortly after initial use of the cell.
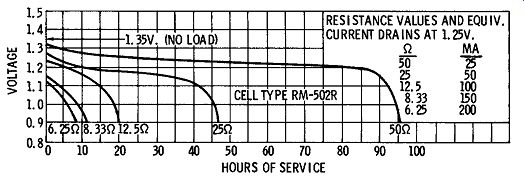
FIG. 5. Voltage output of loaded mercury cells.
ALKALINE CELLS
There are several basic types of alkaline cells, including alkaline manganese, silver oxide-zinc, and silver oxide-cadmium. Of these, the alkaline-manganese is the most widely used in electronic circuits.
An electrochemical action is involved in the alkaline cell, so in this respect it is similar to the carbon-zinc cell. However, the alkaline manganese cell employs an alkaline electrolyte, whereas the carbon zinc uses an acid electrolyte.
The alkaline-manganese cell has an open-circuit terminal voltage of about 1.45 volts; however, it delivers most of its available power below 1.25 volts. Alkaline-manganese cells are capable of a higher energy-discharge rate than zinc-carbon cells of comparable size. They can be continuously discharged without sacrificing electrical capacity.
This is not the case with zinc-carbon cells; where they are used it is desirable to provide recovery time between periods of heavy current discharges.
It is possible, within limitations, to successfully recharge alkaline manganese cells. If it has not been discharged to below 40 percent of its capacity, and if the charging is done at a low rate, a typical alkaline manganese cell may be recharged 50 to 150 times.
-------- Courtesy P. R. Mallory & Co., Inc.
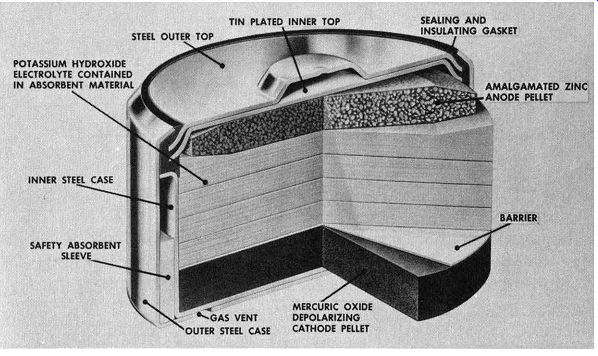
Fig. 6. Construction of typical mercury cell.
SOLAR CELLS
Although the solar cell is not electrochemical in action, it deserves mention since it is becoming a practical source of power. Applications for the solar cell range from sun-powered portable radios to orbiting spacecraft.
FIG. 7 shows the basic construction of a solar cell; it consists of a P-N semiconductor "sandwich." In the absence of illumination, electrons in the N-type material and holes in the P-type material are kept from combining by the "barrier potential" present at the P-N junction. When the junction is illuminated, energy from the light splits valence electrons from some of the atoms, and these produce current in the cell.
FIG. 8 illustrates an application where solar cells are used to charge a nickel-cadmium battery. During periods of sunlight the solar cells provide charging current for the nickel-cadmium battery, which may then be used as a power supply during periods of darkness. The diode prevents discharge of the nickel-cadmium battery back through the solar cell.
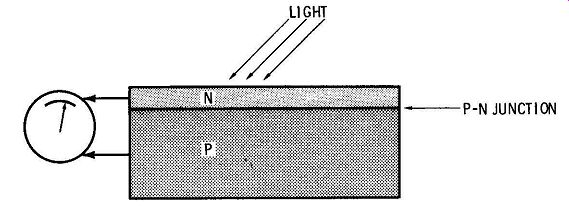
FIG. 7. Operation of a silicon solar cell.
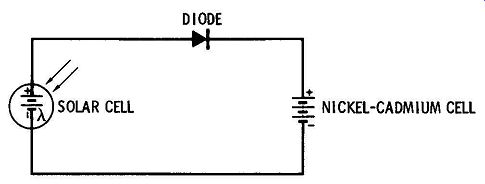
FIG. 8. Solar cell charging a nickel-cadmium cell.

-- A silicon solar cell for the conversion of radiant energy into
electrical energy. Courtesy International Rectifier Corp.
--- Courtesy International Rectifier Corp. Solar power packs are
available with outputs of 3.5 to 16 volts, 30 to 60 milliamperes.
SECONDARY CELLS
The chemical action within the primary cell cannot be reversed; that is, once the chemicals have been exhausted, the cell is no longer capable of generating electricity. Usually this happens because one or more of the reaction products are in a form that removes them from further activity, such as gas that disappears into the atmosphere.
By contrast, the secondary cell has the characteristic that its chemical action may be reversed. Current passing through it in the opposite direction causes a chemical action which restores the reactants to their original condition. Perhaps the most familiar example of the secondary cell is the lead-acid storage battery, which is widely used in automobiles.
Lead-Acid Cell
The following is a simplified description of the chemical reaction that takes place within a lead-acid cell when the cell is delivering cur rent to a load. The sulfuric-acid electrolyte is broken down into positive hydrogen ions and negative sulfate ions. The spongy lead which comprises the negative electrode also dissolves slightly into electrolyte; the positive lead ions thus produced leave behind free electrons which can flow through the external load. The negative sulfate ions combine with the positive lead ions to form lead sulphate, which is deposited on the negative electrode.
The lead peroxide at the positive electrode reacts with the sulfuric acid electrolyte to form positive lead ions; in additional reactions negative hydroxyl ions are produced. The positive ions combine with the negative sulfate ions in the electrolyte to form sulphate, which is de posited on the positive electrode.
During discharge of the lead-acid cell, hydrogen and hydroxyl ions combine as part of the electrochemical reaction to gradually replace part of the sulfuric acid in the electrolyte with water. This results in a lowering of the specific gravity of the electrolyte. It is possible, therefore, to determine the charge of the cell by measuring the specific gravity of its electrolyte with a hydrometer.
When the lead-acid cell is discharged, it may be recharged by sending a current through it in a direction opposite to its discharge cur rent. During this charging process the chemical reactions involved in the discharge cycle are reversed. The lead sulphate which was deposited on the positive electrode during discharge is converted back into lead peroxide, and the negative plate is converted back to spongy lead.
The open-circuit terminal voltage of the lead-acid cell is 2.1 volts.
--------- Courtesy Texas Instruments, Inc. Rechargeable nickel-cadmium
batteries are available in a wide variety of sizes and shapes.
Nickel-Cadmium Cells
The nickel-cadmium cell offers many of the most desirable characteristics of a secondary cell-sealed construction, excellent shelf life, and mechanical ruggedness. The negative electrode is made of a metallic cadmium, the positive electrode is nickel hydroxide, and potassium hydroxide is used for an electrolyte. When the cell is completely discharged, the positive electrode becomes nickel oxide, the negative electrode becomes cadmium hydroxide, and the electrolyte remains unchanged.
The terminal voltage of a fully charged nickel-cadmium cell is 1.2 volts. These cells can be discharged and recharged many times over a period of years before their usefulness is ended.
Silver-Cadmium Cell
The silver-cadmium cell is one of the most efficient-and most expensive-of all secondary cells. Basically it consists of a silver-oxide cathode, a cadmium anode, and an electrolyte of potassium hydroxide. This cell is capable of providing a considerably greater current than the nickel-cadmium cell. The chief drawback is the high cost of the silver used in its construction.